Abstract
Background Mutations in NPM1 (NPM1+) occur in ∼30% of patients with AML. NPM1+ has been associated with favorable outcomes following intensive chemotherapy (IC) and demonstrated positive prognostic value in the absence of concurrent FLT3-internal tandem duplication (FLT3-ITD) mutations. Recently, several studies have reported differences in outcomes in patients with NPM1 mutations based on age and treatment. In this analysis we evaluated treatment patterns and outcomes in AML based on NPM1 mutational status and primary induction therapy in patients who were enrolled in the CONNECT® Myeloid Disease Registry (NCT01688011).
Methods
Patients aged 55 years or older with AML were grouped by known NPM1 status (NPM1+ vs NPM1‒) and induction therapy (non-IC vs IC). Overall survival (OS) and event-free survival (EFS) were evaluated using the Cox model adjusted for age. For the EFS analyses, an event was defined as death, disease progression, or refractory to therapy.
Results
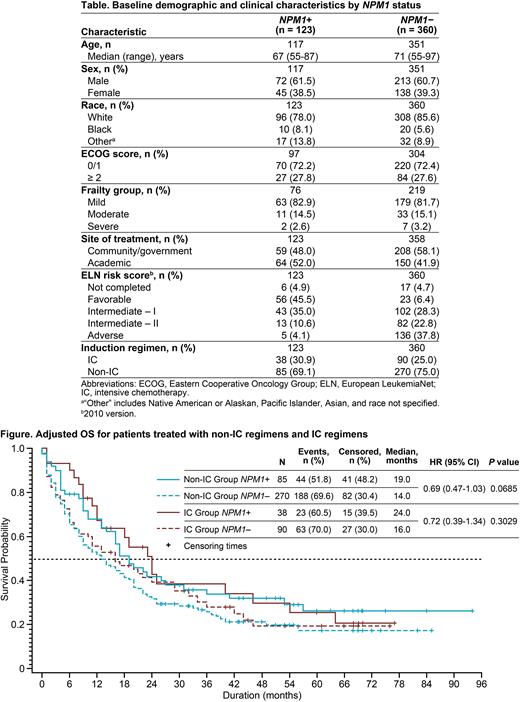
A total of 483 patients with known NPM1 status were assessed (NPM1+, n = 123; NPM1‒, n = 360). Most patients were male (NPM1+, 61.5%; NPM1‒, 60.7%) and white (NPM1+, 78.0%; NPM1‒, 85.6%; Table). Median age, Eastern Cooperative Oncology Group performance status (ECOG PS), and frailty group were similar in the NPM1+ and NPM1‒ groups. Favorable and adverse European LeukemiaNet (ELN) risk scores at baseline were different in the NPM1+ and NPM1‒ groups. The majority of patients received non-IC regimens as induction therapy (NPM1+, 69.1%; NPM1-, 75.0%).
Overall, patients with NPM1+ AML had significantly longer median OS (26 mo) compared with patients with NPM1- AML (15 mo) (HR [95% CI]: NPM1+ vs NPM1- 0.58 [0.44-0.76]; P < 0.01). When analyzed by type of induction regimen (non-IC vs IC), although OS remained numerically longer for patients in the NPM1+ groups, the differences were not statistically significant. Among patients who received non-IC regimens, median OS was 19 and 14 mo in the NPM1+ and NPM1- groups, respectively (Figure). Among patients who received IC regimens, median OS was 24 and 16 mo in the NPM1+ and NPM1- groups, respectively.
There was a significant improvement in EFS for patients in the NPM1+ group vs the NPM1- group (20 mo vs 11 mo; hazard ratio [HR] [95% CI], NPM1+ vs NPM1- 0.61 [0.47-0.80]; P < 0.01). This was also true for patients treated with non-IC regimens (15 vs 11 mo in the NPM1+ and NPM1- groups, respectively; HR [95% CI], NPM1+ vs NPM1- 0.67 [0.46-1.00]; P = 0.048). In contrast with the non-IC treated regimens, in patients treated with IC regimens, NPM1+ status was not associated with significantly longer EFS compared to NPM1- status.
Patients with known NPM1 status were further evaluated for additional mutations and the following differences were noted: the frequency of mutations in tumor protein p53 (TP53) (1.6% vs 18.6%), RUNX1 (0.8% vs 14.8%), ASXL1 (2.4% vs 17.6%) IDH2 (8.9% vs 15.7%), and NRAS/KRAS (8.9% vs 14.3%) was lower in patients with NPM1+ vs NPM1- AML, respectively, whereas the frequency of mutations in FLT3-ITD (35.8% vs 17.1%), FLT3- tyrosine kinase domain (TKD) (14.6% vs 6.2%), DNMT3A (23.6% vs 15.7%), and IDH1 (14.6% vs 8.6%) was higher in patients with NPM1+ vs NPM1- AML, respectively.
Conclusions Overall, patients with NPM1+ AML had longer OS and EFS than patients with NPM1‒ AML. This was observed among patients with NPM1+ AML for both non-IC and IC induction regimens. Patterns of co-mutations differed between the NPM1+ and NPM1- groups and is a likely contributing factor to differences between outcomes.
Disclosures
Maciejewski:Apellis Pharmaceuticals: Consultancy; Alexion: Consultancy. Roboz:Janssen: Consultancy, Other: travel and accommodation expenses, Research Funding; Mesoblast: Consultancy; Actinium: Consultancy; AbbVie: Consultancy, Other: travel and accommodations, Research Funding; CTI: Research Funding; Sandoz: Consultancy, Other: Travel and accommodation expenses; Jazz: Consultancy, Other: travel; Agios: Other: travel, Research Funding; Roche: Consultancy; Bristol Myers Squibb: Consultancy; Otsuka: Consultancy; Onconova Therapeutics: Research Funding; Tensha Therapeutics: Research Funding; Array BioPharma: Other: Travel and accommodation expenses; Sunesis Pharmaceuticals: Other: Travel and accommodation expenses, Research Funding; Clovis Oncology: Other: Travel and accommodation expenses; Agios: Consultancy, Research Funding; Celltrion: Consultancy, Other: Travel and accommodation expenses; Jasper Therapeutics: Consultancy; Eisai: Other: Travel and accommodation expenses; Genentech/Roche: Consultancy, Other: Travel and accommodation expenses; Astex Pharmaceuticals: Consultancy, Other: Travel and Accommodation expenses, Research Funding; Takeda: Consultancy; Daiichi Sankyo: Consultancy; Amphivena Therapeutics: Other: Travel and accommodation expenses, Research Funding; Bayer: Consultancy, Other: Travel and accommodation expenses; Celgene: Consultancy, Other: travel and accommodation expenses, Research Funding; Bristol Myers Squibb: Consultancy; MedImmune: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; Helsinn Therapeutics: Consultancy; Pfizer: Consultancy, Honoraria, Other: Travel and accommodation expenses; Novartis: Consultancy, Other: Travel and accommodation expenses, Research Funding; Amgen: Consultancy; GlaxoSmithKline: Consultancy; Astellas: Consultancy; Karyopharm Therapeutics: Research Funding; Amgen: Consultancy, Other: travel; Mofitt Cancer Center: Research Funding. Seiter:Alexion Pharmaceuticals: Honoraria; Novartis: Honoraria; Jazz Pharmaceuticals: Research Funding; Theradex: Research Funding; Rafael Pharmaceuticals: Research Funding; GlycoMimetics: Research Funding; Takeda: Research Funding; Bristol Myers Squibb: Research Funding; Incyte: Honoraria, Research Funding; Sellas Life Sciences: Research Funding. Scott:Nektar: Other: data and safety monitoring board; Jazz Pharmaceuticals: Other: Advisory Panel; Novartis: Other: Advisory Panel, Research Funding; Alexion: Consultancy; Celgene: Consultancy, Honoraria, Other: Advisor Panel; Bristol Myers Squibb: Consultancy, Honoraria, Other: Advisory Panel, Research Funding; Johnson and Johnson: Other: data and safety monitoring board; Incyte: Consultancy. DeGutis:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kiselev:Juno: Current holder of stock options in a privately-held company; Celgene: Current holder of stock options in a privately-held company; Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company. Yu:BMS: Current Employment, Current equity holder in publicly-traded company, Other: travel and accommodation expenses. McBride:Bristol Myers Squibb: Current Employment. Heydendael:Bristol Myers Squibb: Current Employment. Erba:Trillium Therapeutics: Consultancy; Janssen Oncology: Consultancy; Covance (Abbvie): Consultancy, Other: Independent Review Committee, Research Funding; Novartis: Consultancy, Research Funding, Speakers Bureau; MacroGenics: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; ImmunoGen: Consultancy, Research Funding; Glycomimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Celgene: Consultancy, Other, Speakers Bureau; Astellas Pharma: Consultancy; Amgen: Consultancy, Research Funding; Agios: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy; Kura Oncology: Consultancy; Forma Therapeutics: Research Funding; Gilead/Forty Seven: Research Funding; PTC therapeutics: Research Funding; ALX Oncology: Research Funding; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal